Team:HokkaidoU Japan/Notebook/August25
From 2010.igem.org
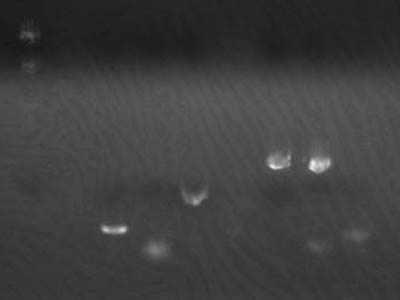
Transformation Results
Colony Check
| Plate | Colonies |
| M 34 | no |
| M 12 | yes |
| I 34 | no |
| I 34 | yes |
| 1-3A 34 | yes |
| 1-3A 12 | yes |
- 1-3A had colonies in lated on mediums containing 34 ug/uL of chloramphenicol
- So the medium and antibiotic consentration seems ok,
- Medium on which M 12 was plated was inoculated with chloramphenicol after solidification so there were some irregularities and colonies on the fringe
- Colonies of 1-3A and 12 were obviously different
→ So we suspected issues in Ligation and DNA concentration bused for transformation
Estimation of 1-3A Consentration
- Added 1 uL of 1-3A into 30 uL of competent cells
- Transformation was successful
- Electrophoresed 1 uL of 1-3A,calculated amount of transformed DNA
→Estimated concentration 2 ng/uL
Ethanol precipitation of pSB1C3 and Ligation
Ethanol precipitation
- Added 8.2 uL of sodium acetate [3 M]
- Added 205 uL of Ethanol [100%] and mixed
- Centrifuge at 15,00rpm, 4C for 10 min
- Discarded supernatant and added 500 uL of Ethanol [70%], mixed
- Centrifuge at 15,00rpm, 4C for 5 min
- Discarded supernatant
- Dried via vacuum desiccator
- Added 8.2 uL of ADW を加え,元の10倍に濃縮する
- Concentration increased 10 fold
Ligation
- Mixed 8.2 uL of DNA solution with the same volume of Ligation Solution
- Added extra 1 uL of T4 ligase
- Incubated at 16C for 30 min
Tomorrow will do aditional Electrophoresis gel extraction and transformation
らおりプライマーで作ったPCR productsのDigestion
前日のPCRから,増やすことのできたパーツのDNA量を求めた
| RBS | 27 ng/uL | 312 bp |
| GFP | 54 ng/uL | 1020 bp |
| dT | 49 ng/uL | 429 bp |
Digestion 反応系
| Reagent | Amount |
|---|---|
| 10x M buffer | 1 uL |
| DW | 4.5 |
| DNA | 2.5 |
| BSA | 1 |
| EcoR I | 0.5 |
| Pst I | 0.5 |
| Total | 10 uL |
→37℃で60分
→ラオリプライマーの真価を電気泳動で確認 "
"