Team:Korea U Seoul/Project
From 2010.igem.org

|
Abstract
Toxic heavy metals such as arsenic, zinc, and cadmium in water are very harmful. Detecting these heavy metals is an important task. So we designed a heavy-metal-detecting E. coli. In order to design the system, we employed two fluorescence proteins (GFP, RFP) and aryl acylamidase as signal reporters. The aryl acylamidase converts a colorless acetaminophen(Tylenol TM) to a brown color substrate. Since the detecting E. coli has three heavy metal promoters, if more than two heavy metals coexist in a solution, the E. coli emit mixed fluorescence, so we simultaneously detect metals. Our goal is to synthetic modules put these three genes for different heavy metals in a row in E. coli and then utilized in the form of a lyophilized powder, which can be stored in a drug capsule to make it portable so that analysis of water is easily processed. We call it a "Capsule Cop".
Introduction
- Pollutant
- A pollutant is a waste material that pollutes air, water or soil, and is the cause of pollution.There are many pollutant all around us. Pollutant may enter ecosystem as the consequence of human activities in the follwing ways. First, unintended relese in course of human activities(e.g., in nuclear accidents, mining operations, shipwrecks, and fires). Second, diposal of waste(e.g., seweage, industrial effluents). And last, Deliberate application of biocides(e.g., pest control and vector control)
- Metal
- There are many types of pollutatns. Especially, Metal pollutant attracted our attention. A metal is defined by chemists as being an element that has a characteristic lustrous appearance, is good conductor of electrocity, and generally enters chemical reactions as positive ions or cations. There are a few examples of localized metal pollution resulting from natural wethering of ore bodies. However, in most cases, metals become pollutants where human activities, mainly through mining and smelting, releases them from the rocks in which they were deposited during volcanic activity or subsequent erosion and relocates them into situations where they can cause environmental demage.
- That is clear that human activity is responsible for the global movement of
Cadmium, Lead, Zinc, Mercury, Arsenic. And these metals can be pollutant when its concentration exceeds a threshold value in a particular envionmental compartment. The compartment is the whole planet, but compartments can be individual organism or thing as small as single cells or even organelles within cell. Especially, Cadmium, Zinc, Arsenic can be harmful when these metals exist high concentration.
- Cadmium
- Cadmuim is accumulated in the liver and kidneys of mammals. The cadmium poisoning caused softening of the bones and kidney failure. The one of sympoms of cadmuim poisoning are proteinuria (excretion of proteins in the urine) as result of the breakdown of the kidney cells when cadmium levels exceed a critical concentration. Itai-itai disease (イタイイタイ病,"ouch ouch sickness")is also one of sympoms of cadmium, that was the documented case of mass cadmium poisoning in Toyama Prefecture, Japan, starting around 1912. One of the main effects of cadmium poisoning is weak and brittle bones. Spinal and leg pain is common, and a waddling gait often develops due to bone deformities caused by the cadmium. The pain eventually becomes debilitating, with fractures becoming more common as the bone weakens. Other complications include coughing, anemia, and kidney failure, leading to death.
- Zinc
- In regions contaminated heavily with metals, concentrations of zinc can exceed 2% of the dry weight of the hepatopancreas of woodlice. In these circumstances, detoxification capacity of the organ is eventually exceeded, the cells begin to break down, and the woodlouse becomes moribund because of zinc poisoing.
- Arsenic
- During the 18th, 19th, and 20th centuries, a number of arsenic compounds have been used as medicines, including arsphenamine (by Paul Ehrlich) and arsenic trioxide (by Thomas Fowler). Arsphenamine as well as Neosalvarsan was indicated for syphilis and trypanosomiasis, but has been superseded by modern antibiotics. Arsenic trioxide has been used in a variety of ways over the past 500 years, but most commonly in the treatment of cancer. The US Food and Drug Administration in 2000 approved this compound for the treatment of patients with acute promyelocytic leukemia that is resistant to ATRA. It was also used as Fowler's solution in psoriasis. Recently new research has been done in locating tumours using arsenic-74 (a positron emitter). The advantages of using this isotope instead of the previously used iodine-124 is that the signal in the PET scan is clearer as the body tends to transport iodine to the thyroid gland producing a lot of noise.
- The high affinity of arsenic(III) oxides for thiols is usually assigned as the cause of the high toxicity. Thiols in the form of cysteine residues, are situated at the active sites of many important enzymes.
- These metals are very hamful when their concentration exceeds a threshold value. So we tried to figure out how we can detect concentration of these metals using micro-organism. If we detect concentration of these metals early, we can reduce damage to some degree.
Design
To sense heavy metals, we select promoters which are expressed in the presence of heavy metals. These promoters encode proteins maintaing cell conditions resistant to heavy metals. ParsR is promoter to arsenic and PzntA is for zinc.
Low concentrations can have devastating effects, interfering with processes in the cell. Because of the similarity to phosphate, sometimes arsenate is mistaken for phosphate, which is how it is introduced into living organisms, including E. coli, by the phosphate uptake system. Other molecules such as As(III) can also be introduced into the cells by various membrane transporters. Cells have effective defenses to deal with elevated levels of arsenic variants, which are under the control of metal sensitive promoters. The transcription is mostly regulated by proteins binding to, or releasing from the promoter site, like ArsR.
ArsR negatively controls the expression of the genes involved in arsenical and antimonite metals resistance, whose expression is induced in the presence of these metals.
In case of cadmium, there is no promoters encoding resistant to cadium directly. So we select Promoter yodA.
At low cadmium concentrations, cells are able to adapt and resume growth after a period of stasis. Promoter yodA is only transiently activated during cadmium-induced growth inhibition. Aryl acylamidase is an enzyme that acts on the amide bond between aryl- and acyl groups. The most typical reaction is the hydrolysis of an anilide, producing a carboxylate and aniline, which is also reversible.
Acetaminophen(Tyrenol) is an aryl acyl compound having amide bond is hydrolyzed by the enzyme and produce acetate and p-aminophenol.
Because p-Aminophenol have red-purple color, aryl acylamidase gene will apply to reporter gene.
Since aryl acylamidase gene(mAAA, [http://partsregistry.org/wiki/index.php?title=Part:BBa_K400628 BBa_K400628]) is not registered to iGEM, we registered this gene.
Result
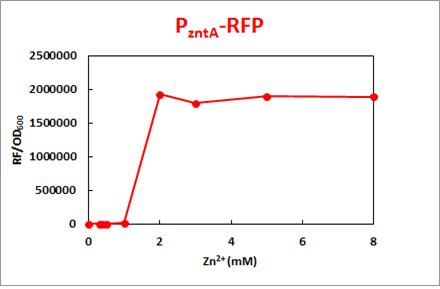
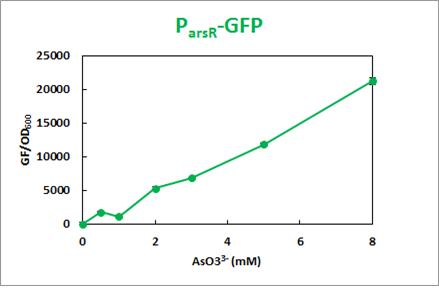
RFP sore was detected in between 1.0mM-2.0mM of Zn2+,whereas subtle emission below 1.0mM, and GFP showed gradual increase in signal with the concentration of arsenic.
Discussion
Last year, we designed a Integrated Heavy Metal Detection System for simultaneous detection of multiple heavy metals such as arsenic, zinc, and cadmium in E. coli. We successfully tested the promoters and aryl acylamidase worked well with the promoter. This year we put three of the genes together in one plasmid and made a composite part. We also submitted mAAA(part: [http://partsregistry.org/wiki/index.php?title=Part:BBa_K400628 BBa_K400628]) which is put next to promoter yodA and a member of composite part([http://partsregistry.org/wiki/index.php?title=Part:BBa_K400000 BBa_K400000]).
This system can be portablized in a capsule by lyophilization.
Freeze dry
| The process of freeze-drying(lyophilization) involves the removal of water vapour by vacuum
sublimation from the frozen state. Freeze-drying has been widely used for many years as one of the preferred method for long-term preservation. Freeze-drying is particularly suitable for collections which supply cultures on demand because of its broad applicability, economy of storage space and ease of distribution. Freeze-drying is the most technologically complex of the preservation methods in use. It is labour intensive and requires the highest level of technical skill. The method is suitable for many types of microorganisms including most bacteria, yeast, sporing-fungi and some viruses, algae and protozoa. |
| One of the major advantages of freeze-drying is that the ampoules are particularly suitable as a means of distributing cultures because the viability and intengrity of the ampoules the airmail services. |
| A disadvantage of freeze-drying is the relativelt high capital cost of commercial equipment. However, the principle of freeze-drying is quite simple and for small scale operations a laboratory freeze-dryer can be manufactures readily by any competent workshop. All that is a vacuum pump, a moisture trap, an evacuation chamber and a drying manifold. |
In this way, 50% of the E. coli is available for detection. To develop our project, we need to upgrade our part. The standard of heavy metal permission is listed below(water quality standard, 2011).
| Heavy metal | Standard permission |
|---|---|
| Arsenic | 0.01mg/L = 1.3×10^(-4) mM |
| Zinc | 3mg/L = 4.6×10^(-2) mM |
| Cadmium | 0.005mg/L = 4.4×10^(-5) mM |
Reflecting this standard, we need detection level close to the standard. What we can do is upgrade our promoter to a more sensitive one or find a way to amplify the signal with newly designed parts.
For instance, according to the result, the graph shows that the promoter zntA gives a sore of RFP in between Zn2+ concentration 1.0-2.0mM. Since the detection level should be near the standard, the promoter should be about 40-fold more sensitive. We could figure out some ways to develop the system.
Reference
Puskárová A, Ferianc P, Kormanec J, Homerová D, Farewell A, Nyström T. Regulation of yodA encoding a novel cadmium-induced protein in Escherichia coli. Microbiology. 2002 Dec;148(Pt 12):3801-11.
H.W.Doelle. Microbial process development. World scientific. 1994. pp.64-65.
C.H. Walker, S.P. Hopkin, R.M. Sibly, D.B Peakall, " Principles of Ecotoxicology", Taylor&Francis, 2006, pp3, 23, 54, 125-126
 "
"

