Team:HokkaidoU Japan/Notebook/September14
From 2010.igem.org
(Difference between revisions)
(→Team:HokkaidoU_Japan/ProtocolsEthanol precipitation) |
|||
| Line 88: | Line 88: | ||
→Incubated at 37C for 120 min<br> | →Incubated at 37C for 120 min<br> | ||
→Added Sample Buffer 10 uL and electrophoresed | →Added Sample Buffer 10 uL and electrophoresed | ||
| - | * | + | * pSB1C3 band wasn't visible |
→ Other 2 were gel extracted | → Other 2 were gel extracted | ||
* Diluted that final volume would be 40 uL | * Diluted that final volume would be 40 uL | ||
| Line 95: | Line 95: | ||
[[Image:HokkaidoU Japan 20100914b.jpg|200px|right|thumb|Electrophoresis after ethanol precipitation]] | [[Image:HokkaidoU Japan 20100914b.jpg|200px|right|thumb|Electrophoresis after ethanol precipitation]] | ||
| - | * Added ethanol acetate[ | + | * Added ethanol acetate[3 M] 4 uL to 40 uL of previously purified DNA solution |
| - | * Added EtOH[100%] 44 uL | + | * Added EtOH[100%] 44 uL froze in dry ice |
| - | * Melted and centrifuged at | + | * Melted and centrifuged at 15000 rpm, 4C 5 min |
* Discarded supernatant and added EtOH[70%] 100 uL rinsed | * Discarded supernatant and added EtOH[70%] 100 uL rinsed | ||
* Centrifuged at 15,996rpm, 4C 5 min | * Centrifuged at 15,996rpm, 4C 5 min | ||
| Line 134: | Line 134: | ||
|- | |- | ||
|style="border-top:1px solid #996;"|'''Total''' | |style="border-top:1px solid #996;"|'''Total''' | ||
| - | |style="border-top:1px solid #996;"|'''12.4 uL' | + | |style="border-top:1px solid #996;"|'''12.4 uL''' |
|} | |} | ||
*50 uL were transformed by electroporation and remaining 50 uL by heat shock (chemical) | *50 uL were transformed by electroporation and remaining 50 uL by heat shock (chemical) | ||
Latest revision as of 08:20, 27 October 2010
Digestion and ligation of pSB1C3, araC Promoter and GFP
Digestion
Used pSB1C3 which was PCRed september 6th. DNA concentration was 2256 ng/uL. So diluted 20 times.
| Reagent | Amount |
|---|---|
| 10x M buffer | 5 uL |
| DW | 34 |
| pSB1C3 | 1 |
| BSA | 5 |
| EcoR I | 2 |
| Pst I | 3 |
| Total | 50 uL |
| Reagent | Amount |
|---|---|
| 10x M buffer | 5 uL |
| DW | 27 |
| Promoter | 8 |
| BSA | 5 |
| EcoR I | 3 |
| Spe I | 2 |
| Total | 50 uL |
| Reagent | Amount |
|---|---|
| 10x M buffer | 5 uL |
| DW | 32 |
| GFP | 3 |
| BSA | 5 |
| Xba I | 4 |
| Pst I | 1 |
| Total | 50 uL |
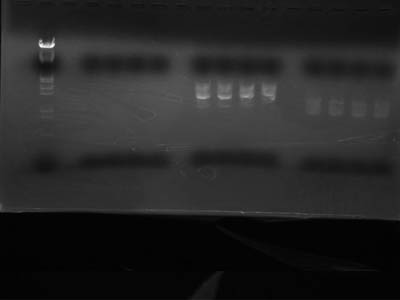
→Incubated at 37C for 120 min
→Added Sample Buffer 10 uL and electrophoresed
- pSB1C3 band wasn't visible
→ Other 2 were gel extracted
- Diluted that final volume would be 40 uL
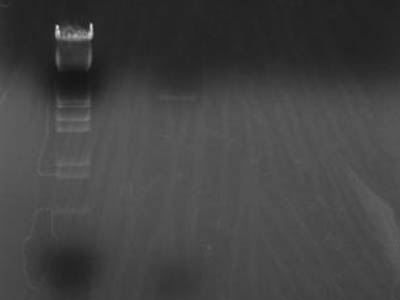
Ethanol precipitation
- Added ethanol acetate[3 M] 4 uL to 40 uL of previously purified DNA solution
- Added EtOH[100%] 44 uL froze in dry ice
- Melted and centrifuged at 15000 rpm, 4C 5 min
- Discarded supernatant and added EtOH[70%] 100 uL rinsed
- Centrifuged at 15,996rpm, 4C 5 min
- Discarded supernatant and dried
- Diluted in 2 uL of TE each
- Anticipated concentrations
promoter: 125 ng/2uL
GFP: 106 ng/2uL
- pSB1C3 was cut(EcoR1, Pst1) on September 6th
- Did ethanol precipitation and electrophoresed to check concentration
- It was about 20 ng/uL
→Used 2 uL of it
Ligation
| Reagent | Amount |
|---|---|
| pSB1C3 | 2 uL |
| promoter | 2 uL |
| GFP | 2 uL |
| Mighty Mix | 6 uL |
| T4 ligase | 0.4 uL |
| Total | 12.4 uL |
- 50 uL were transformed by electroporation and remaining 50 uL by heat shock (chemical)
 "
"