Team:Slovenia/PROJECT/biosynthesis/violacein
From 2010.igem.org
| (4 intermediate revisions not shown) | |||
| Line 37: | Line 37: | ||
<br> | <br> | ||
<h2>Background</h2> | <h2>Background</h2> | ||
| - | Ever since 1882, violacein has been notable as the most obvious characteristic of a soil bacterium <em>Chromobacterium violaceum</em> due to its deep purple color. Violacein is insoluble in water and due to its hydrophobicity retained within cells. Biosynthesis of violacein has been as well as its biological activities were investigated in | + | Ever since 1882, violacein has been notable as the most obvious characteristic of a soil bacterium <em>Chromobacterium violaceum</em> due to its deep purple color. Violacein is insoluble in water and due to its hydrophobicity retained within cells. Biosynthesis of violacein has been as well as its biological activities were investigated in detail (Duran et al., 2007). Violacein has antibacterial, antiviral, antiparasite, antitumorogenic and many other biological activities, which makes the violacein pigment a very good candidate for further research and industrial production. In addition to potential medical applications, violacein is also used as a natural purple dye.<br> |
<h2>Violacein biosynthetic pathway</h2> | <h2>Violacein biosynthetic pathway</h2> | ||
| - | Genes for violacein biosynthesis (scheme below) are arranged in an operon consisting of <em>vioA, vioB, vioC, vioD</em> and <em>vioE</em>. VioA is an FAD dependent L-tryptophan oxidase, which generates an IPA imine. The latter is a substrate for VioB, a hemoprotein oxidase, which converts the IPA imine into a dimer. VioE is a key enzyme in the violacein biosynthetic pathway that acts by converting the IPA imine dimer to | + | Genes for violacein biosynthesis (scheme below) are arranged in an operon consisting of <em>vioA, vioB, vioC, vioD</em> and <em>vioE</em>. VioA is an FAD dependent L-tryptophan oxidase, which generates an IPA imine. The latter is a substrate for VioB, a hemoprotein oxidase, which converts the IPA imine into a dimer. VioE is a key enzyme in the violacein biosynthetic pathway that acts by converting the IPA imine dimer to intermediates, which can be taken over by VioD and VioC. VioD and VioC are FAD dependent monooxygenases that hydroxylate these compounds to form violacein and deoxyviolacein. The genes coding for the five enzymes of the violacein biosynthetic pathway have already been succesfully expressed in <em>E.Coli</em>. In <em>E.Coli</em> an additional side reaction occurs, producing a green pigment called deoxychromoviridans, which is produced before the action of VioD and VioC. We aimed to link all violacein biosynthetic enzymes into an arranged pathway assembled along the program DNA with the goal to increase the yield of the desired product. |
[[Image:SLOviolacein8.jpg|thumb|center|600px|'''The violacein biosynthetic pathway''' (Balibar and Walsh, 2006). Enzyme-catalysed biosynthetic transformation of tryptophan into violacein is shown. The purple line indicates the desired biosynthetic flux, which we aimed to achieve by introduction of a DNA program into the cells that produce chimeric biosynthetic enzymes linked to different zinc fingers. This reaction pathway should favor violacein synthesis over the side reaction leading to deoxychromoviridans.]]<br> | [[Image:SLOviolacein8.jpg|thumb|center|600px|'''The violacein biosynthetic pathway''' (Balibar and Walsh, 2006). Enzyme-catalysed biosynthetic transformation of tryptophan into violacein is shown. The purple line indicates the desired biosynthetic flux, which we aimed to achieve by introduction of a DNA program into the cells that produce chimeric biosynthetic enzymes linked to different zinc fingers. This reaction pathway should favor violacein synthesis over the side reaction leading to deoxychromoviridans.]]<br> | ||
| Line 53: | Line 53: | ||
<br><br> | <br><br> | ||
<h2>Results</h2> | <h2>Results</h2> | ||
| - | <em>E. coli</em> containing chimeric enzymes and either correct DNA program (123456), scrambled DNA program or no program were grown for extended time. In addition, The E.coli strain carrying plasmids with violacein operon (Part:Bba_K274002), prepared by the 2009 iGEM team Cambridge, was also grown for comparison. <br> | + | <em>E. coli</em> containing chimeric enzymes and either correct DNA program (123456), scrambled DNA program or no program were grown for extended time. In addition, The ''E.coli'' strain carrying plasmids with violacein operon ([http://partsregistry.org/Part:BBa_K274002 Part:Bba_K274002]), prepared by the 2009 iGEM team Cambridge, was also grown for comparison. <br> |
Bacteria containing chimeric proteins were able to produce violacein (no program), demonstrating that the <strong>addition of zinc finger domains does not interfere with their enzymatic activity</strong> (Figure 1). However, the production of violacein was delayed compared to the cultures that contained DNA programs. | Bacteria containing chimeric proteins were able to produce violacein (no program), demonstrating that the <strong>addition of zinc finger domains does not interfere with their enzymatic activity</strong> (Figure 1). However, the production of violacein was delayed compared to the cultures that contained DNA programs. | ||
Latest revision as of 03:59, 28 October 2010
Contents |
State of the art
Violacein is a compound that is synthesized in five steps from tryptophan. Transfer of the biosynthetic pathway from Chromobacterium violaceum allows production of violacein by E.Coli. Parts for the complete violacein biosynthetic pathway are available in the Registry and succesful production of violacein has been demonstrated by the Cambridge 2009 iGEM team. Besides purple violacein, the same biosynthetic pathway also produces a green side product deoxychromoviridans.Aims
We wanted to test whether our idea can be applied to the real world application. We examined the advantages of DNA-guided protein assembly on the violacein pathway. Our goal was to construct chimeric biosynthetic enzymes and a DNA program coding for the correct order of biosynthetic enzymes.Achievements
We designed and constructed a modified violacein biosynthetic pathway composed of five chimeric proteins with violacein biosynthetic enzymes and different zinc fingers. Introduction of DNA program coding for the correct order of chimeric proteins into bacteria expressing violacein these chimeric enzymes resulted in 6-fold improved yield of violacein, in comparison to DNA coding for a scrambled order of biosynthetic pathway. Additionally, this arrangement resulted in a significant decrease of an unwanted side product deoxychromoviridans. These resultates demonstrate in a very convincing way the advantages of DNA scaffold platform for biosynthesis.
Background
Ever since 1882, violacein has been notable as the most obvious characteristic of a soil bacterium Chromobacterium violaceum due to its deep purple color. Violacein is insoluble in water and due to its hydrophobicity retained within cells. Biosynthesis of violacein has been as well as its biological activities were investigated in detail (Duran et al., 2007). Violacein has antibacterial, antiviral, antiparasite, antitumorogenic and many other biological activities, which makes the violacein pigment a very good candidate for further research and industrial production. In addition to potential medical applications, violacein is also used as a natural purple dye.
Violacein biosynthetic pathway
Genes for violacein biosynthesis (scheme below) are arranged in an operon consisting of vioA, vioB, vioC, vioD and vioE. VioA is an FAD dependent L-tryptophan oxidase, which generates an IPA imine. The latter is a substrate for VioB, a hemoprotein oxidase, which converts the IPA imine into a dimer. VioE is a key enzyme in the violacein biosynthetic pathway that acts by converting the IPA imine dimer to intermediates, which can be taken over by VioD and VioC. VioD and VioC are FAD dependent monooxygenases that hydroxylate these compounds to form violacein and deoxyviolacein. The genes coding for the five enzymes of the violacein biosynthetic pathway have already been succesfully expressed in E.Coli. In E.Coli an additional side reaction occurs, producing a green pigment called deoxychromoviridans, which is produced before the action of VioD and VioC. We aimed to link all violacein biosynthetic enzymes into an arranged pathway assembled along the program DNA with the goal to increase the yield of the desired product.

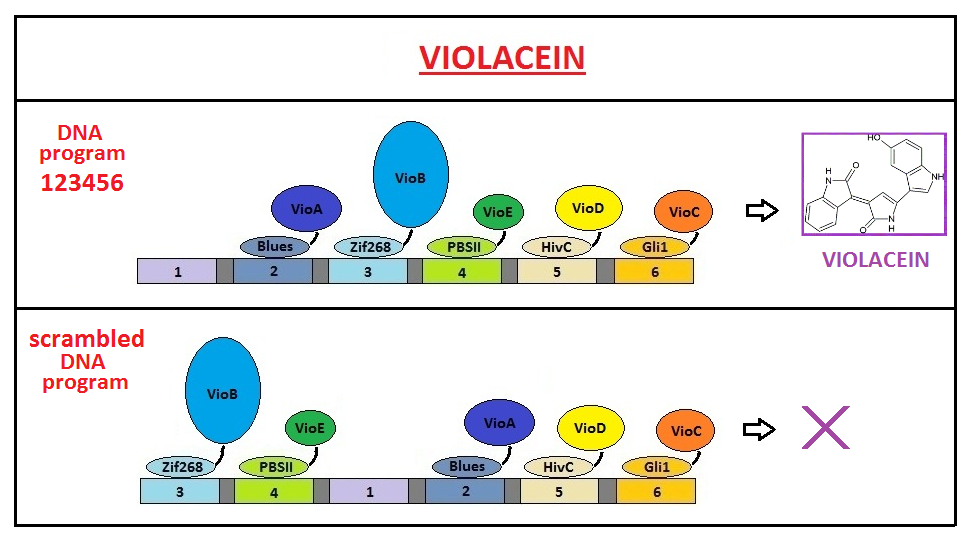
Design of a DNA-guided biosynthetic pathway
We designed five fusion proteins by combining different zinc fingers and appropriate violacein biosynthetic enzymes. Since we wanted to create an in vivo system for violacein production, we expressed them in E. coli. Our goal was to show improvement of violacein biosynthesis by DNA program (scaffold) for which we predicted it would increase the proximity of fusion proteins and arrange their order according to the biosynthetic order of reactions. To demonstrate the significance of corect order of biosynthetic enzymes for violacein production, we compared production of violacein in the presence of correct DNA program (123456) to production in the presence of DNA programs with scrambled order for binding of chimeric proteins (341256; see the scheme below).
Results
E. coli containing chimeric enzymes and either correct DNA program (123456), scrambled DNA program or no program were grown for extended time. In addition, The E.coli strain carrying plasmids with violacein operon ([http://partsregistry.org/Part:BBa_K274002 Part:Bba_K274002]), prepared by the 2009 iGEM team Cambridge, was also grown for comparison.
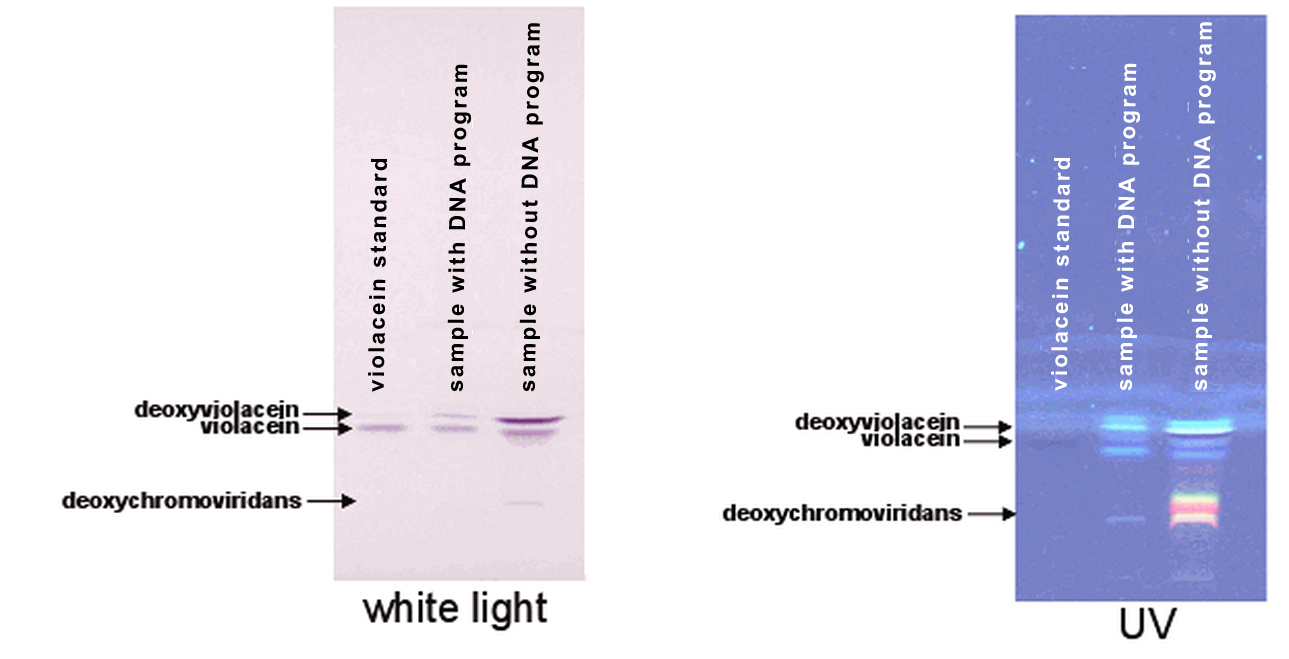
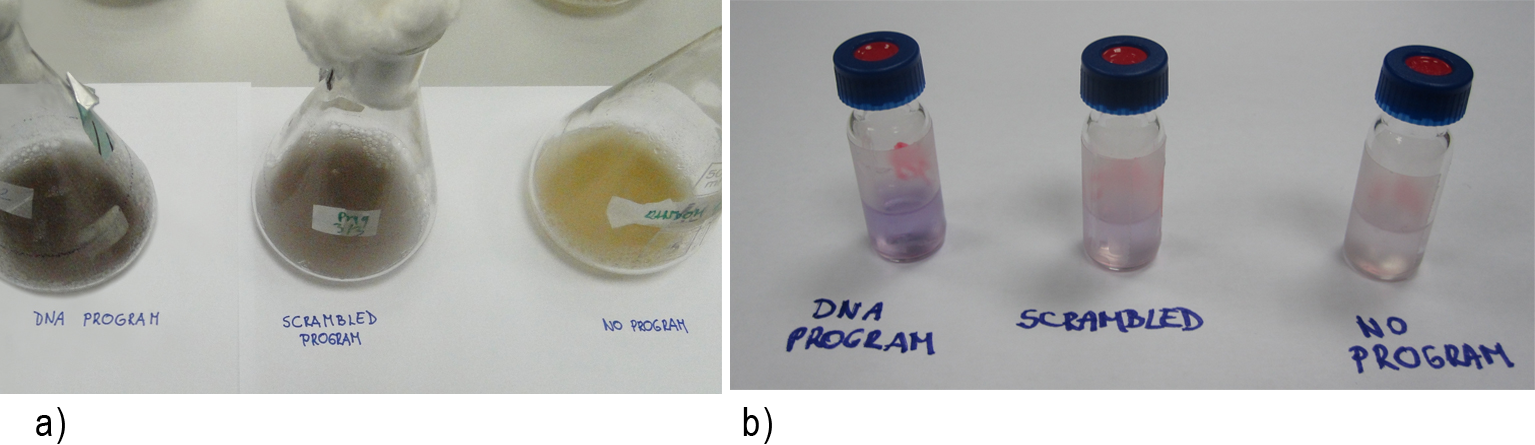
Bacteria containing chimeric proteins were able to produce violacein (no program), demonstrating that the addition of zinc finger domains does not interfere with their enzymatic activity (Figure 1). However, the production of violacein was delayed compared to the cultures that contained DNA programs.
We further analyzed the extracts using more sensitive methods, e.g. thin-layer chromatography (TLC) with densitometry, mass spectrometry (for a detailed description of the protocols see the page "Methods"). Results show that in additon to violacein and deoxyviolacein, exctracts of bacterial cultures with enzymes without zinc finger domains contained many more side products (Figure 3, line 3), particularly deoxychromoviridans. The identity of all compounds was determined by mass spectrometry and absorbance spectra. However, a significant decrease of deoxychomoviridans and deoxyviolacein product formation was detected, when program DNA was present (Figure 2, compare lanes 2 and 3).
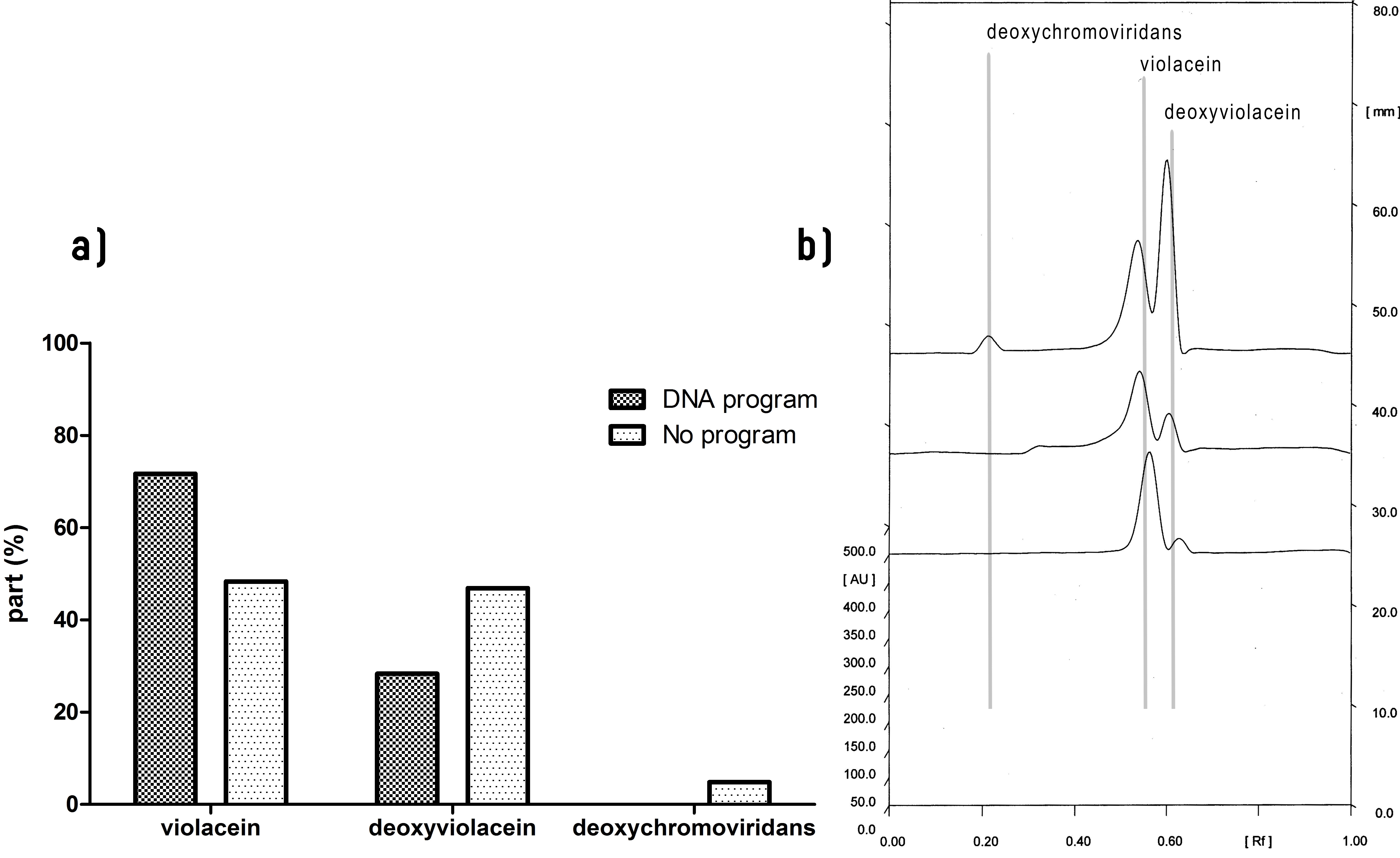
Most important, the yield of violacein when program DNA was present has improved ~6-fold and ~2 fold, in comparison to yield when no or scrambled DNA program was present, respectively (Figure 4).
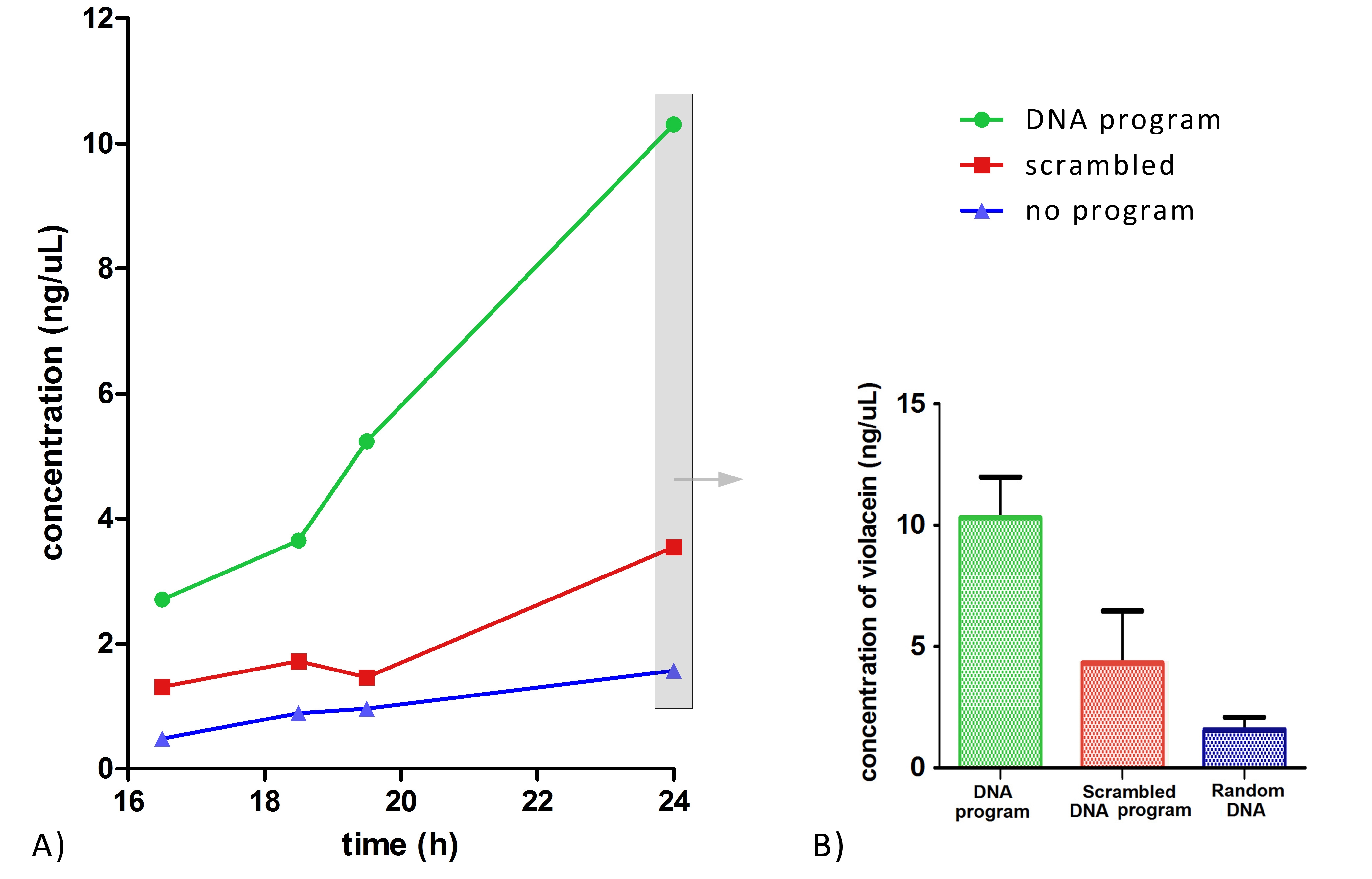
These results fully confirm the idea of DNA-guided biosynthetic assembly and its usefulness for the improvement of the biosynthetic flux. In addition to the anticipated improvement of the yield, we were very pleased to observe significant suppression of the side products (deoxychromoviridans and deoxyviolacein), which probably also contributed to the increase of the final yield of violacein.
Although the theoretical increase of the speed inthe biosynthetic pathway composed of a five reactions, the final yield depends on many different factors, including the rate limiting step, availability of the cofactors, the initial substrate - tryptophane, etc. We did not have time to perform any optimizations with respect to strains, growth media, temperature etc., therefore the maximum yield of violacein production could be significantly higher. However the experiment was repeated more than three times with similar results.
Duran N., Justo G.Z., Ferreira C.V., Melo P.S., Cordi L., Martins D. 2007. Violacein: Properties and biological activities. Biotechnology and Applied Biochemistry, 48: 127-133
Balibar CJ, Walsh CT. 2006, In vitro biosynthesis of violacein from L-tryptophan by the enzymes VioA-E from Chromobacterium violaceum, Biochemistry, 45:15444-57.
 "
"