Transformation of pUC119, RFP, pSB1C3 and RFP
Parts information
| DNA
| Concentration
| Length
| Amount
|
| RFP (1-5A)
| 80 ng/uL
| 1000 bp
| 26 uL
|
| pSB1C3
| 20 ng/uL
| 2000 bp
| 35 uL
|
| pUC119(pre cut)
| 10 ng/uL
| 3000 bp
| 28 uL
|
Digestion
| Reagent
| Amount
|
| RFP (1-5A)
| 26 uL
|
| DW
| 9 uL
|
| 0.1% BSA
| 5 uL
|
| 10x M Buffer
| 5 uL
|
| EcoR I
| 2 uL
|
| Pst I
| 3 uL
|
| Total
| 50 uL
|
| Reagent
| Amount
|
| pSB1C3
| 33 uL
|
| DW
| 2 uL
|
| 0.1% BSA
| 5 uL
|
| 10x M Buffer
| 5 uL
|
| EcoR I
| 2 uL
|
| Pst I
| 3 uL
|
| Total
| 50 uL
|
→Incubated at 37C for 60 min
→Adde 10 uL 6x Sample buffer and electrophoresed
To check concentration lanes 2 through 5 contained 1uL of DNA solution
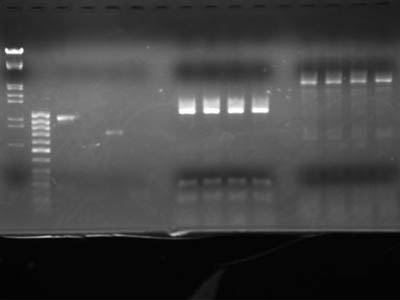
Electrophoresis of digestion product
| Lane
| DNA
|
| 1
| λ/Hind III, EcoR I
|
| 2
| 50 base ladder
|
| 3
| Heat shock promotor
|
| 4
| RBS
|
| 5
| RFP protein coding
|
| 6
| double Terminator
|
| 7
| blank
|
| 8
| RFP reporter (1-5A) 15 uL each
|
| 9
|
| 10
|
| 11
|
| 12
| blank
|
| 13
| pSB1C3 15 uL each
|
| 14
|
| 15
|
| 16
|
| 17
| blank
|
Bands were exised (210 mg and 220 mg acordingly)
Final volume was made to be 20 uL, with disregard to promega's protocol
At this point concentrations were: RFP 104 ng/uL,pSB1C3 33 ng/uL.
Ligation
| Reagent
| Amount
|
| RFP
| 1.5 uL
|
| pSB1C3
| 1.5 uL
|
| ligation Kit I
| 3 uL
|
| T4 ligase
| 0.5 uL
|
| Total
| 6.5 uL
|
| Reagent
| Amount
|
| RFP
| 1.5 uL
|
| pSB1C3
| 1.5 uL
|
| Mighty Mix
| 3 uL
|
| T4 ligase
| 0.5 uL
|
| Total
| 6.5 uL
|
| Reagent
| Amount
|
| RFP
| 0.8 uL
|
| pUC119
| 4 uL
|
| ligation Kit I
| 4.8 uL
|
| T4 ligase
| 0.5 uL
|
| Total
| 10.5 uL
|
| Reagent
| Amount
|
| RFP
| 0.8 uL
|
| pUC119
| 4 uL
|
| Mighty Mix
| 4.8 uL
|
| T4 ligase
| 0.5 uL
|
| Total
| 10.5 uL
|
→Incubated 16C for min
→
Transformation
 "
"






