Team:Brown/Notebook/July22
From 2010.igem.org
Thursday, July 22, 2010
Redo of yesterday's failed digest
Followed same protocol as yesterday, created the same master mix
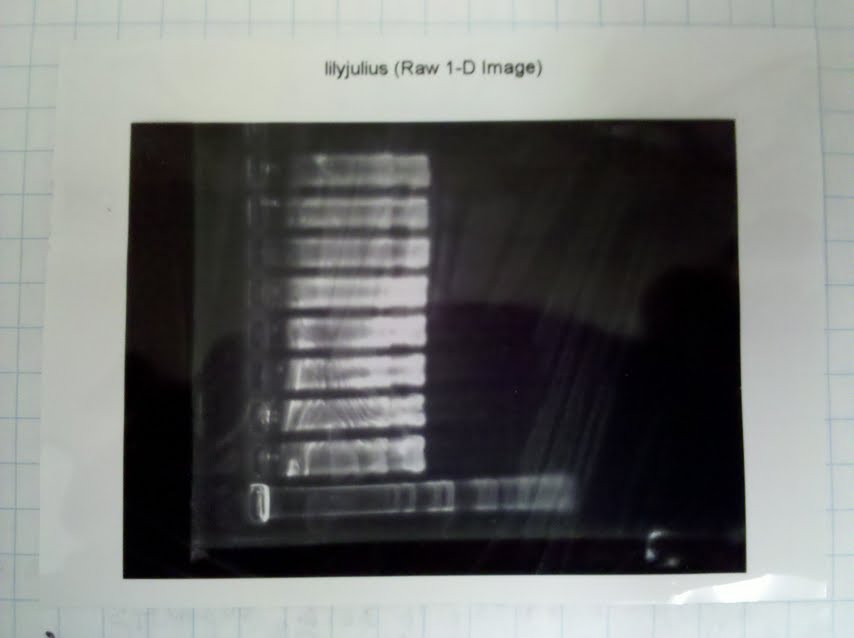
- Cast and ran a 1% agarose gel
- Again, resulted in a lot of streaking :/
- Loaded 20ul (entire reaction)
- Used 1kb+ ladder. The DNA seems to be around 3800bp, which suggests that it is possible that our pGEM did incorporate the WillRS insert but the one enzyme cut and the other did not (resulting in a linear piece of DNA). The other possibility is that we loaded too much DNA into each well. This would have made the DNA run slower, so it looks like 3.8 even though it is actually 3.
- Optimal amount of DNA to run for a gel is 0.5ug
Observations on plates from last night's transformation
- All Kan plates are covered in solid lawn/film, akin to null plates. Smells yeasty (oh no, contamination!)
- Parts AraC+pBad+SupD #1 & #2 and NaHR+pSal all have isolated colonies growing, but some show contamination
- Kan+amp+ plates with Lovtap+Lovtap2 appear to have some growth, no contamination.
- However, no controls were made to test the Kan
Adrian suggested that instead of cutting with NcoI and BamHI, we cut with EcoRI b/c there are EcoRI sites near where our inserts would have been (near the TA overhang) so we can more easily confirm that we have our insert. He also recommended wthat we use less DNA, and to dot he digest rxn at 50ul (by adding more buffer and dH2O).
Plasmid miniprep from L.Lactis (from abandoned miraculin expt)
From Vaccine Protocols; Second ed.; Robinson, Hudson, Cranage; 2003; Human Press
"Resuspend in resuspension buffer supplemented with lysozyme and mutanolysin (to a final concentration of 5mg/ml and 100U/ml respectively). Incubate the resuspended cells at 37C for 15 minutes"
- Prepared stock solution of 125mg/ml lysozyme
- Prepared stock soln of 1KU/mL mutanolysin
Miniprep
- Pelleted 3ml L.Lactis in MRS (spun at 5.5k RPM for 3min)
- Resuspended in 215ul P1, 25ul 1KU/ml mutanolysin, 10ul 125mg/ml lysozyme
- Incubated at 37C for 15min
- Followed standard miniprep protocol from Qiagen kit
Nanodrop data: 210.9 ng/ul with appropriate 260/280 and 260/230 :D
Primers Received Today
From Invitrogen:
- Tat-linker_FW/Rev
- RFC25AraC_FW/Rev
- RFC25LacI_FW/Rev
Rehydrated primers to 100uM stock and created 10uM working dilutions
PCR of LacI A/B, pWillRS linear (x2)
20ul total volume:
- 10ul master mix 2x
- 1ul FW primer @10.5uM
- 1ul Rev primer @10.5uM
- 1ul DNA
- 7ul H2O
Used standard iGEM program, melt temp of 55C for primers
Second attempt at rehydrating J00421 from 2008 paper registry
- Used 10ul of EB
- Used all of the rehydrated DNA in transformation of XL1Bs
- Transformation incubated on ice for 40min
- Heatshock, iced, added 100ul LB
- Plated and incubated overnight @37C
 "
"