Team:Brown/Notebook/August11
From 2010.igem.org
Wednesday, August 11, 2010
===Transformation of Lovtwo ligation into XL1B
- RFP control: 5ul of DNA (at 1/1000 dilution) into LB Amp+
- pGEM-Lovtwo: 8ul DNA into LB Amp+
Plated for blue-white selection with 50ul xGAL (20mg/ml stock), 2ul 1M IPTG
Rehydrated primers
Rehydrated primers to 100uM
- CI fwd - 14.24nm -> 142.4ul dH2O
- CI rev - 11.24nm -> 112.4ul dH2O
- Mnt fwd - 17.49nm -> 174.9ul dH2O
- Mnt rev - 16.21nm -> 162.1ul dH2O
- AraC fwd - 12.28nm -> 122.8ul dH2O
- AraC rev - 16.46nm -> 164.6ul dH2O
PCR of AraC, Mnt, CI
For each reaction, 50ul total volume:
- 2ul forward primer
- 2ul reverse primer
- 1ul template DNA
- 20ul dH2O
- 25ul Master mix (Promega)
Note - CI miniprep DNA was found stored at 4C so we moved it to 20C with the others
Resulting constructs:
- Bgl prefix-RBS-AraC-Bgl suffix
- Bgl prefix-RBS-CI-Bgl suffix
- Bgl prefix-RBS-Mnt-terter-Bgl suffix
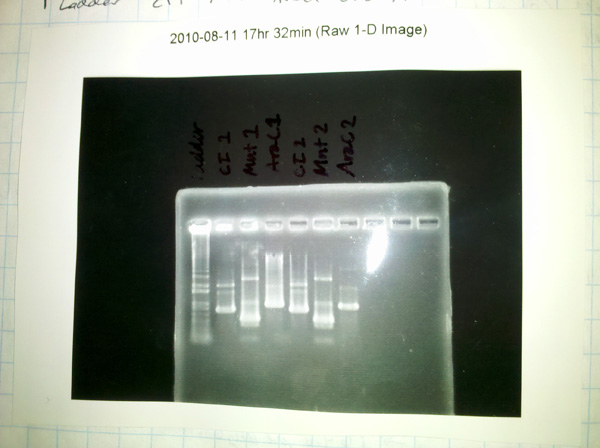
Ran gel of results:
All the reactions produced additional undesired products. Mnt 1+2 have potential primer dimers...
Found potential vector for carrying TatLinker and LacI/AraC
J13002 was rehydrated from 2010 plate 1 well 13B in 10ul dH2O
This was transformed into XL1, plated overnight.
PCR of RFC25 sites onto miniprepped AraC (C0080)
20ul total volume:
- 10ul of 2x master mix
- 1ul fwd primer (0.05uM end concentration)
- 1ul rev primer (0.05uM end concentration)
- 1ul DNA
- 7ul H2O
Result was placed into James' box in the freezer
Overnights
Took overnight plate of BL21 transformed with J04421, grew overnight liquid cultures from harvested colonies.
Placed the lone white colony from yesterday's lovtwo transformation and incubated @37C
 "
"